The field of bioprinting technology has evolved significantly over the years, offering tremendous potential in various areas of healthcare, regenerative medicine, and tissue engineering.
The Evolution of Bioprinting Technology
Bioprinting is a cutting-edge technology that involves the layer-by-layer deposition of living cells, bio-inks, and biomaterials to create complex three-dimensional (3D) structures. Here's an overview of the evolution of bioprinting technology:
-
Early Beginnings
Bioprinting can trace its roots back to the mid-20th century when the concept of 3D printing was first introduced. Early experiments involved printing simple structures using cell-laden gels.
-
Development of Bioinks
The key to Bioprinting's success lies in the development of suitable bioinks, which are materials that can carry and support living cells. Researchers have worked on improving the biocompatibility and printability of these inks.
-
Inkjet Bioprinting
In the late 1990s, inkjet bioprinting emerged as one of the first techniques. It involved using modified inkjet printers to deposit cell-laden droplets onto a substrate. This technique allowed for high-resolution printing but had limitations in printing complex structures.
-
Extrusion Bioprinting
Extrusion-based Bioprinting became popular in the early 2000s. It uses a syringe-based system to extrude bioink layer by layer. This method gained recognition for its ability to print a broader range of biomaterials, including hydrogels and cell aggregates.
-
Stereolithography and Laser-Assisted Bioprinting
Stereolithography and laser-assisted bioprinting techniques use light or laser energy to solidify bio-inks layer by layer selectively. These methods offer high precision and speed, making them suitable for creating complex structures.
-
Bioprinting for Tissue Engineering
As the technology advanced, Bioprinting started being used to create tissues and organs. Researchers began working on printing functional tissues like blood vessels, skin, and even small organs. This development holds immense promise for organ transplantation and regenerative medicine.
-
3D Bioprinting for Drug Testing
Bioprinting technology is also employed in drug testing and personalized medicine. Researchers can print miniature organ models that mimic the functions of real organs, allowing for more accurate drug testing and disease modeling.
-
Integration with Stem Cells
Stem cells have become an integral part of bioprinting technology. The ability to differentiate stem cells into various cell types has allowed for the creation of more complex and functional tissues.
-
Bioprinting of Complex Organs
In recent years, breakthroughs have been in bioprinting entire organs like hearts, livers, and kidneys. Although these advancements are still experimental, they offer hope for addressing the global shortage of donor organs.
-
Emerging Materials and Biofabrication
Bioprinting is evolving with the development of new biomaterials and fabrication techniques. These innovations are expanding the possibilities of Bioprinting, making it more versatile and accessible.
-
Regulatory and Ethical Considerations
The evolution of bioprinting technology is accompanied by regulatory and ethical challenges, such as ensuring the safety and efficacy of bioprinted products, addressing intellectual property issues, and defining ethical boundaries.
Bioprinting technology has come a long way from its early experimental stages. It has the potential to revolutionize medicine and healthcare by providing patient-specific treatments, reducing the need for organ transplants, and advancing our understanding of human biology. As the technology continues to evolve, it will be critical to address the associated ethical, legal, and regulatory issues to maximize its positive impact on society.

What is 3D Bioprinting?
3D Bioprinting is an innovative technology that combines three-dimensional (3D) printing techniques with biological materials, such as living cells and biocompatible bioinks, to create complex, functional, and customized biological structures, tissues, and even organs. This cutting-edge approach is at the intersection of biotechnology, regenerative medicine, and 3D printing, and it holds immense promise for various applications in healthcare and biomedical research.
Key features and components of 3D Bioprinting include:
Bioinks: These specialized materials serve as the "ink" in Bioprinting. Bioinks can consist of living cells, biomaterials, growth factors, and other biological components. They are carefully formulated to provide a suitable environment for cell growth, viability, and tissue development.
Printing Technology: 3D bioprinters are equipped with specialized print heads and nozzles designed to deposit bio-ink in a controlled and precise manner. Standard printing technologies include extrusion-based Bioprinting, inkjet-based Bioprinting, and stereolithography-based Bioprinting.
Layer-by-Layer Deposition: Similar to traditional 3D printing, 3D Bioprinting builds structures layer by layer. The bioink is deposited layer by layer, and in the case of living tissues, cells are arranged in a way that mimics their natural organization.
Complex Tissue and Organ Fabrication: 3D Bioprinting has the potential to create complex tissues, like blood vessels, skin, and cartilage, as well as functional organs, such as the heart, liver, and kidney. These structures can be custom-designed to match the patient's needs and anatomy.
Biological Relevance: One of the main advantages of 3D Bioprinting is that it can closely replicate the microarchitecture and composition of natural tissues, making it an invaluable tool for regenerative medicine, drug testing, and disease modeling.
Materials Used in 3D Bioprinting
In 3D Bioprinting, various materials are used to create the bioinks and support structures necessary to fabricate complex biological tissues and organs. These materials provide the environment for cell growth, organization, and tissue development. Here are some of the critical materials commonly used in 3D Bioprinting:
Cells
Primary Cells: Cells derived directly from the patient's tissues, offering the potential for personalized treatments.
Stem Cells: Pluripotent or multipotent stem cells, such as induced pluripotent stem cells (iPSCs) or mesenchymal stem cells (MSCs), can differentiate into various cell types.
Cell Lines: Established cell lines that are immortal and can be used for large-scale production of tissues for research or transplantation.
Biomaterials
Hydrogels: These are water-based materials that provide a supportive and biocompatible scaffold for cells. Standard hydrogels include alginate, agarose, gelatin, and hyaluronic acid.
Extracellular Matrix (ECM) Components: ECM components like collagen, fibrin, and laminin are often used to mimic the natural environment of cells in tissues.
Synthetic Polymers: Biodegradable synthetic polymers like polylactic acid (PLA), polyglycolic acid (PGA), and polycaprolactone (PCL) can be used to create structural support or as bioink components.
Decellularized Tissue Matrices: Tissues are stripped of cells to leave behind the ECM, which can be used as a bioink or scaffold for cell attachment and growth.
Growth Factors and Cytokines
Growth factors, such as vascular endothelial growth factor (VEGF) and transforming growth factor-beta (TGF-β), are often added to bioinks to stimulate cell differentiation and tissue development.
Crosslinking Agents
These substances are used to solidify the bio-ink after deposition. Standard crosslinking methods include chemical crosslinking using agents like glutaraldehyde or UV light exposure for photopolymerization.
Support Materials
Sometimes, a temporary support material may be used to create complex 3D structures. These supports are typically removable after printing. Examples include sacrificial hydrogels.
Nutrient and Oxygen Supply
In addition to the bio-inks and support materials, it's essential to provide a continuous supply of nutrients and oxygen to the printed tissues. This can be achieved through a perfusion system that circulates the culture medium through the bioprinted structure.
Biocompatible Inks
Specialized inks are designed for use in the 3D bioprinter's print heads or nozzles, ensuring that the cells and biomaterials remain viable and functional during the printing process.
The selection of materials depends on the specific application and the type of tissue or organ being produced. Researchers and bioprinting experts continue to explore and develop new materials to improve the accuracy, biocompatibility, and functionality of 3D bioprinted constructs. The goal is to create bioprinted tissues and organs that closely mimic the properties of natural biological structures for a wide range of biomedical and clinical applications.

Applications in Healthcare
3D Bioprinting has a wide range of applications in the healthcare industry, revolutionizing how we approach medicine.
Tissue Engineering and Regenerative Medicine
3D Bioprinting has a wide range of applications in tissue engineering and regenerative medicine, offering innovative solutions to various medical challenges. Here are some of the critical applications within these fields:
Organ Transplantation: 3D Bioprinting is set to bring about a sea change in organ transplantation. Researchers are diligently working towards constructing fully functional organs, such as kidneys, livers, and hearts, tailored specifically for individual patients. This advancement could drastically reduce reliance on donor organs, diminish the chances of organ rejection, and boost the accessibility of vital treatments for survival.
Tissue Replacement and Repair: Bioprinting can produce tissues and structures suitable for transplantation or implantation, including skin grafts, bone grafts, and cartilage implants. This is particularly advantageous for patients with tissue damage, injuries, or abnormalities.
Wound Healing and Skin Substitutes: 3D Bioprinting enables the creation of artificial skin and wound-healing constructs. These constructs can help in the treatment of burn victims, individuals with chronic wounds, and those in need of skin grafts.
Vascular Tissue and Blood Vessels: Bioprinted blood vessels and vascular tissue can address cardiovascular diseases and improve the outcomes of surgeries and interventions, like coronary artery bypass procedures.
Dental and Craniofacial Applications: Bioprinting creates dental implants, artificial teeth, and custom-made craniofacial implants. It helps patients who require dental or facial reconstruction.
Orthopedic Applications: 3D Bioprinting is employed in orthopedics to create bone grafts and custom implants for joint replacements. These implants can be tailored to match the patient's anatomy precisely.
Ophthalmology: Researchers are working on bioprinting corneal tissue and structures for corneal transplants, potentially restoring vision in individuals with corneal disorders.
Neural Tissue and Nervous System Repair: 3D Bioprinting fabricates neural tissue constructs, such as nerve guides and neural scaffolds. These can assist in nerve regeneration and the treatment of spinal cord injuries and neurodegenerative diseases.
Drug Testing and Disease Modeling: 3D bioprinted tissue models, like liver, heart, and lung constructs, are used for drug testing, toxicity screening, and disease modeling. They offer a more accurate representation of human biology, reducing the need for animal testing and potentially accelerating drug development.
Personalized Medicine: Bioprinting allows for the creation of patient-specific tissues and organs. This personalization can enhance the success of transplants, minimize the risk of immune rejection, and improve treatment outcomes.
Research and Education: 3D bioprinted tissues are valuable tools for scientific research, medical training, and education. They allow researchers and students to study human biology, disease mechanisms, and surgical techniques in a controlled and ethical manner.
Cosmetic and Aesthetic Medicine: Bioprinting is also explored for reconstructive and enhancement procedures in cosmetic and aesthetic medicine.
3D Bioprinting continues to advance, with ongoing research and development efforts to improve the quality, scalability, and clinical translation of bioprinted tissues and organs. While challenges remain, the technology's potential to transform tissue engineering and regenerative medicine is highly promising.

Custom Implants and Prosthetics
3D Bioprinting offers several applications for creating custom implants and prosthetics, providing personalized solutions to individuals with specific medical or anatomical needs. These applications can enhance the functionality, comfort, and quality of life for patients in various medical scenarios. Here are some of the critical applications:
Custom Orthopedic Implants: 3D Bioprinting is used to manufacture personalized orthopedic implants for patients with bone injuries or orthopedic conditions. These implants can be designed to fit the patient's anatomy precisely, providing a better, more stable fit and improving the overall success of joint replacements, such as hip and knee implants.
Dental Implants and Prosthetics: Bioprinting is employed in dentistry to create custom dental implants, crowns, bridges, and dentures. The ability to design these structures to match the patient's oral anatomy improves the comfort and aesthetics of dental restorations.
Craniofacial Implants: Patients with craniofacial defects or those needing facial reconstruction following trauma, surgery, or congenital conditions can benefit from custom craniofacial implants. Bioprinting allows for precise and patient-specific solutions, improving both form and function.
Customized Ear Prosthetics: For individuals with congenital or acquired ear deformities or losses, 3D Bioprinting can create customized ear prosthetics that closely resemble natural ears in shape and appearance.
Ocular Prosthetics: Patients with eye injuries or congenital eye defects can receive custom 3D bioprinted ocular prosthetics, which offer a more natural look and fit than traditional glass eye prosthetics.
Limbs and Prosthetic Devices: While traditional prosthetics are often customized, 3D Bioprinting allows for even greater customization of prosthetic limbs. Prosthetic hands, arms, legs, and other devices can be designed to match the individual's limb size, shape, and functional requirements.
Cochlear Implants: Bioprinting can create custom-fit cochlear implants, enhancing the hearing experience for individuals with hearing impairments.
Spinal Implants and Supports: For patients with spinal injuries or conditions, custom 3D bioprinted spinal implants, such as intervertebral disc replacements or spinal supports, can be created to improve spinal stability and mobility.
Breast Implants: In the field of reconstructive surgery, 3D Bioprinting can be applied to create personalized breast implants for breast cancer survivors who have undergone mastectomies.
Maxillofacial Prosthetics: Patients who have lost parts of their maxillofacial region due to cancer, accidents, or congenital disabilities can benefit from custom 3D bioprinted maxillofacial prosthetics, such as nasal or palate prostheses.
3D bioprinting technology, combined with advanced imaging techniques like CT scans and MRI, enables healthcare professionals to create implants and prosthetics tailored to each patient's unique anatomy. This personalization results in better fit, improved function, increased patient satisfaction, and a higher quality of life for those needing these medical devices.
Drug Development and Testing
3D Bioprinting has a significant impact on drug development and testing by providing more physiologically relevant and reliable tissue models for pharmaceutical research. These applications enhance the drug discovery process, reduce costs, and increase the accuracy of drug testing. Here are some of the critical applications of 3D Bioprinting in drug development and testing:
Disease Modeling: 3D bioprinted tissues and organoids can mimic the microenvironment and complexity of human tissues, making them valuable tools for studying various diseases, including cancer, neurological disorders, and cardiovascular conditions. Researchers can create disease-specific models to understand disease mechanisms better and test potential treatments.
Drug Efficacy Testing: 3D bioprinted tissue models allow pharmaceutical companies to test the efficacy of drug candidates more accurately. These models can provide insights into how a drug interacts with specific tissue types, helping to identify promising candidates early in the drug development process.
Toxicity Screening: Bioprinted tissues assess the safety and potential toxic effects of new drugs. By exposing these tissues to candidate drugs, researchers can identify adverse reactions or side effects that may not be apparent in traditional 2D cell cultures or animal models.
Pharmacokinetics and Pharmacodynamics (PK/PD) Studies: 3D bioprinted tissues can study drug absorption, distribution, metabolism, and excretion (ADME) properties in a more physiologically relevant context. It enables a better understanding of a drug's behavior in the human body.
Personalized Medicine: Bioprinted tissues can be created using a patient's cells, allowing personalized drug testing. Its approach can help identify the most effective treatment options for individual patients and reduce the risk of adverse reactions.
High-Throughput Screening: 3D bioprinting technology can be used for high-throughput drug screening, enabling the rapid testing of many drug candidates. It accelerates the drug development process and reduces costs.
Rare Disease Research: 3D Bioprinting provides a valuable platform for researching rare diseases and developing treatments for conditions with limited treatment options.
Development of Targeted Therapies: The ability to create complex tissue models enables the development of targeted therapies that specifically address the unique characteristics of specific diseases or patient populations.
In Vitro Tumor Models: 3D bioprinted tumor models replicate the microenvironment of tumors more accurately than traditional methods. It is crucial for developing and testing cancer treatments.
Blood-Brain Barrier Models: Bioprinted blood-brain barrier models are used to study drug transport across this critical barrier, aiding in developing drugs for neurological conditions and brain disorders.
3D Bioprinting is becoming an integral part of the drug development process, helping researchers and pharmaceutical companies make informed decisions about the safety and efficacy of potential drugs. These bioprinted tissue models bridge preclinical testing and human clinical trials, ultimately leading to safer and more effective medications for various medical conditions.

Challenges and Ethical Considerations
3D Bioprinting is a transformative technology with the potential to revolutionize healthcare and regenerative medicine. Still, it also brings with it a set of challenges and ethical considerations that need to be addressed. Here are some of the key challenges and ethical concerns associated with 3D Bioprinting:
Challenges
Biocompatibility: Ensuring that the bioprinted materials and structures are fully biocompatible with the human body remains a significant challenge. Materials used in Bioprinting must not trigger immune responses or adverse reactions in recipients.
Vascularization: Creating functional blood vessels within bioprinted tissues and organs is crucial for their survival and proper functionality. Achieving adequate vascularization remains a complex challenge.
Cell Viability: Maintaining cell viability throughout the bioprinting process is essential for the success of the resulting tissues or organs. Printing techniques and materials must be optimized to minimize cell damage.
Long-term Functionality: Ensuring that bioprinted organs and tissues remain functional over an extended period is challenging. It's vital to assess their long-term durability and performance.
Scalability: Scaling up the bioprinting process to produce organs for widespread clinical use is a significant challenge. Achieving consistency and efficiency in large-scale production is necessary to meet the demand for transplantable organs.
Regulatory Approval: Developing a regulatory framework for bioprinted organs and tissues is a complex process. Bioprinted products must meet rigorous safety and efficacy standards to gain regulatory approval.
Ethical and Legal Issues: The question of who owns the rights to bioprinted tissues and organs and how intellectual property is managed is a complex issue. Additionally, issues related to patenting bioprinting techniques and bioproducts are still evolving.
Resource and Cost Constraints: The resources required for 3D Bioprinting, including specialized equipment, skilled personnel, and quality control processes, can be costly. Finding ways to make bioprinted products more affordable is an ongoing challenge.
Ethical Considerations
Informed Consent: Obtaining informed consent is crucial when using a patient's cells to create bioprinted tissues or organs. Patients should be fully aware of how their cells will be used and for what purposes.
Equity and Access: Ensuring equitable access to bioprinted organs and tissues is an ethical concern. The technology should not exacerbate existing healthcare disparities.
Patient Privacy: Protecting patient privacy and the security of their genetic and medical data is essential, especially when using patient-specific cells for Bioprinting.
Human Dignity: The creation and use of bioprinted organs and tissues should respect the inherent dignity of human life. Ethical considerations may arise in cases where Bioprinting involves the manipulation of human embryos or fetal tissue.
Transparency and Accountability: Maintaining transparency in the bioprinting process and holding responsible parties accountable for any ethical breaches or safety concerns is paramount.
Environmental Impact: Consideration should be given to the environmental impact of Bioprinting, including the use of materials, waste disposal, and energy consumption.
Cultural and Religious Perspectives: Bioprinting may raise ethical concerns that differ among cultures and religions. Understanding and respecting these varying perspectives is essential.
Unintended Consequences: Ethical concerns may arise from unforeseen consequences of bioprinting technology, such as potential misuse or unintended health risks.
Addressing these challenges and ethical considerations requires ongoing collaboration among scientists, clinicians, ethicists, policymakers, and the public. Establishing clear guidelines, regulations, and ethical frameworks will ensure that 3D Bioprinting benefits society while minimizing potential risks and ethical dilemmas.
Recent Breakthroughs in 3D Bioprinting
3D Bioprinting is a transformative technology in biomedicine, allowing for the creation complex biological structures and potentially revolutionizing the healthcare industry. Here are a few recent breakthroughs in 3D Bioprinting:
Printed Skin: Researchers from Rensselaer Polytechnic Institute in New York have developed a way to 3D print living skin, complete with blood vessels. It is a significant step forward in creating grafts that are more like the skin our bodies produce naturally.
3D Printed Corneas: Scientists from Newcastle University in the UK have developed the world's first 3D printed human corneas, potentially solving the shortage of available eye donors and helping millions regain their sight.
Bioinks: Researchers are constantly developing new types of bioinks – the materials used in 3D Bioprinting. For instance, a team at the University of Utah has developed a new bioink that makes printing more complex and diverse tissue types possible.
3D Printed Organs: A team at Tel Aviv University in Israel has 3D printed a small, vascularized heart using a patient's cells and biological materials. It was the first time anyone had successfully engineered and printed an entire heart replete with cells, blood vessels, ventricles, and chambers.
Cancer Research: Researchers at Queen Mary University of London have successfully 3D printed human brain structures for cancer research. It will allow for better research and understanding of brain cancer and could lead to better treatments.
High-resolution Cell Printing: Researchers from the University of Stuttgart, Germany, developed a high-resolution bioprinting process that produces structures with a resolution of 10 μm, close to the size of most human cells. It allows for more precise and detailed structures to be printed.
These breakthroughs in 3D bioprinting technology promise exciting medical developments, potentially leading to significant improvements in patient care and treatment.
Conclusion
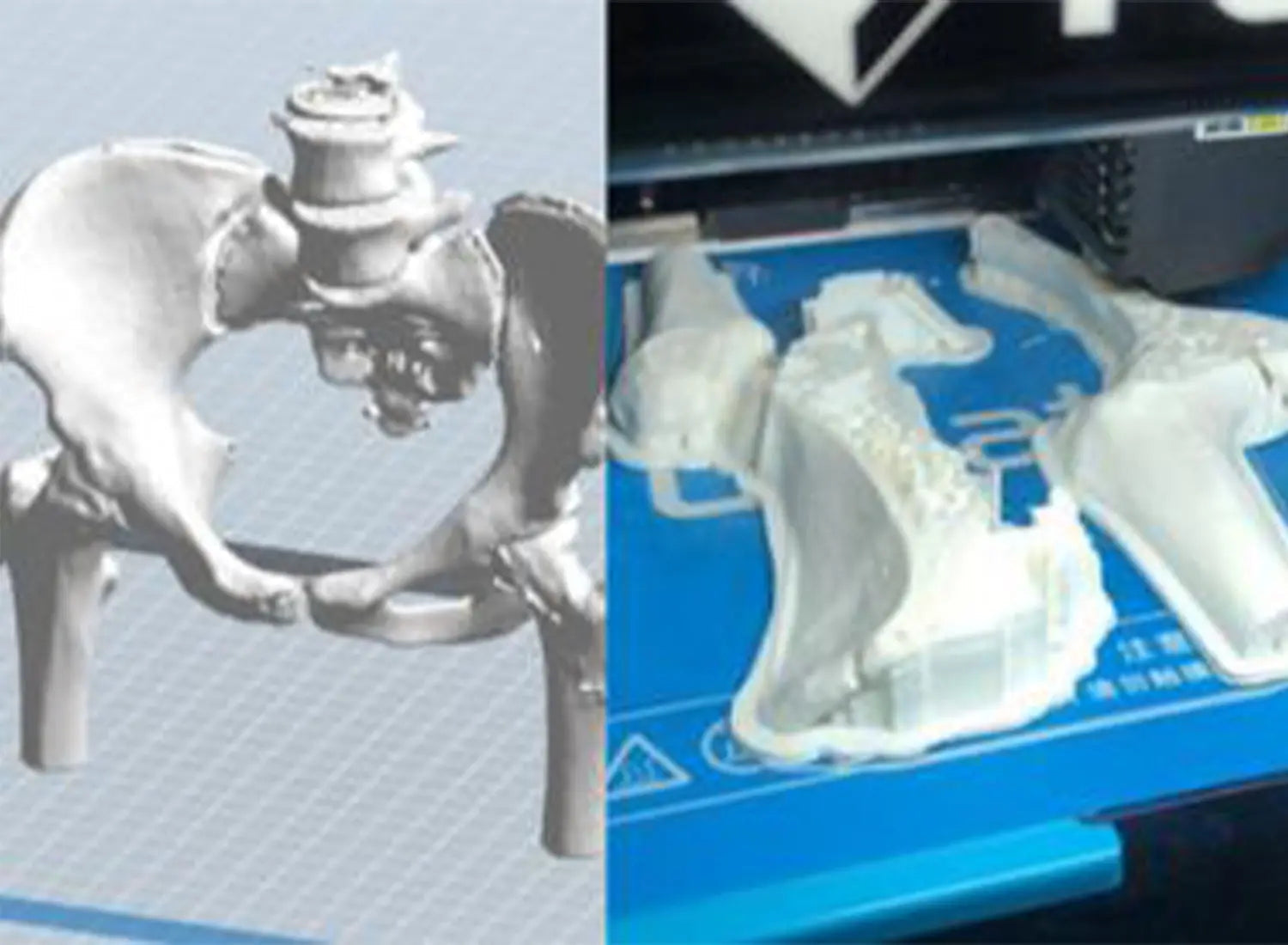
Innovations in 3D bioprinting techniques are taking us closer to a future where organ shortages will be a thing of the past and personalized medicine will be the norm. While challenges and ethical considerations remain, the potential to save lives and improve healthcare is undeniable. With ongoing research and technological advancements, the horizon of 3D Bioprinting seems boundless. Flashforge 3D printers are also widely used in 3D bioprinting, especially dentistry.